Last Updated on: January 19, 2024
Edited By: Alfred
Research
Scientists are conducting a randomized, controlled phase III trials globally among men who were suffering from metastatic CRPC and need first line docetaxel chemotherapy.
Objective
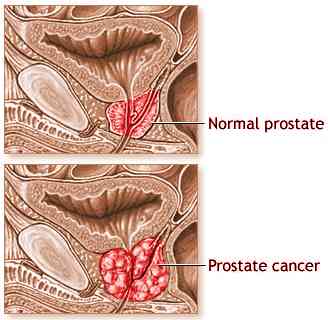
This trail is being conducted to set as a first line of treatment for prostrate cancer.
Study
For the purpose of this study, the patients were randomly distributed and received two forms of treatments
- Treatment I- Docetaxel + custirsen
- Treatment II ‘ Docetaxel
Difference between the two treatments- type II treatment is the gold method of treatment i.e anti-mitotic chemotherapy. This form of treatment is recommended for patients who are suffering from advanced form of prostrate cancer and have already been through the phase of anthracycline-based chemotherapy which did not show the desired result. The type I combine the above docetaxel and custirsen to treat prostrate cancer. Custirsen is a form of antisense oligonucleotide which reduces clusterin production. Clusterin is a form of a protein which inhibits apoptosis. It helps to destroy tumor cells by decreasing their apoptotic threshold and helps the cancer cells react to the different anticancer therapies. The basic aim of the trial is to figure out if the survival rate in the custirsen treatment (type I) is longer than type II treatment. The phase II trials demonstrated that there is 49 % reduction in the death rate and the average survival rate in Type I treatment was 23.8 % as compared to the Type II which was 16.9 % months. Researchers believe that if this third phase III trial validates the previous two phase three trials, type I type of treatment could bring a revolution in the world of oncology as it offered patients a chance to prolong their survival and live a better quality of life. Prostrate cancer is one of the few form of cancers that can have multiple therapies with continuous research on newer therapies. So far, curtirsen has been given the nod from the FDA to go ahead with the trial and the research ‘Synergy’ is being conducted through special Protocol Assessment (SPA) process.

Comments are closed.